The war on counterfeit drugs and substandard medical products and technologies in the country has received a major boost.
This is after the Pharmacy and Poisons Board has adopted new technology as it seeks to ensure drug quality in the sector.
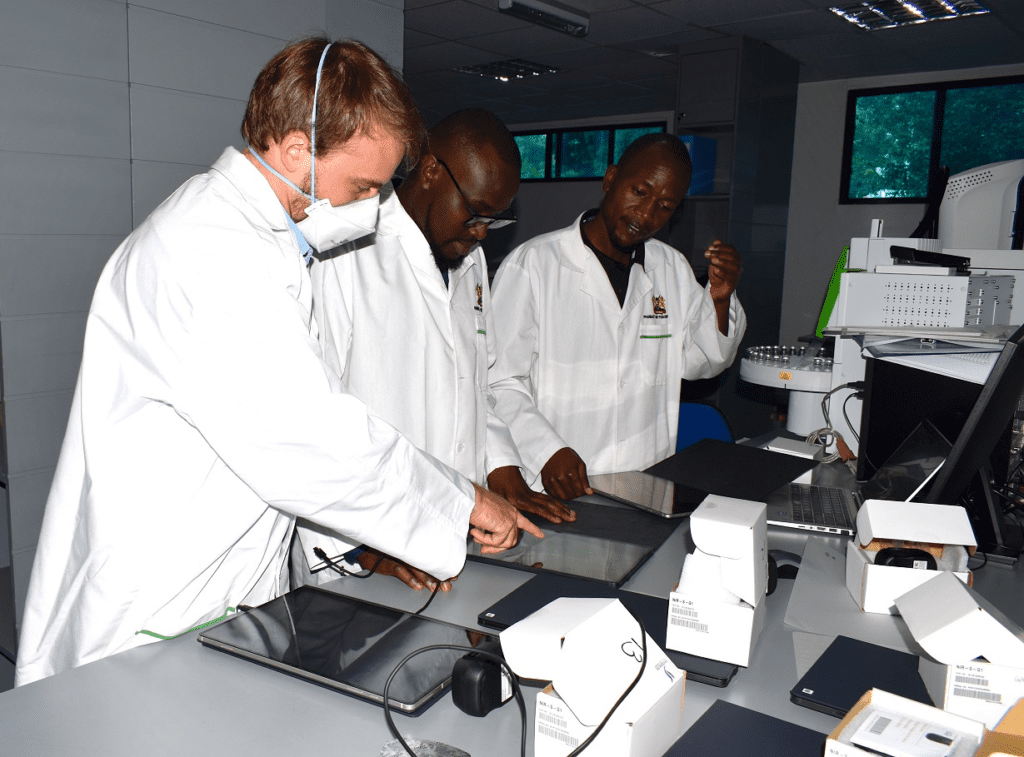
The Pharmacy and Poisons Board (PPB) has introduced cutting-edge technology to combat substandard and falsified medical products.
In a statement issued on Wednesday, December 11, the board announced the implementation of Near Infrared Technology (NIR), known as Pillscan, which aims to strengthen its quality control systems. The advanced tool will ensure the safety, quality, and efficacy of medicines in the Kenyan market.
The PPB highlighted that Pillscan was acquired from Global Health Labs in the U.S. with support from the Global Fund. Its deployment is being executed in partnership with Missions for Essential Drugs and Supplies (MEDS).
“The Pharmacy and Poisons Board, in collaboration with MEDS, has commenced the implementation of advanced Near Infrared Technology (NIR), known as Pillscan,” the statement read. “Acquired from Global Health Labs USA with support from the Global Fund, this cutting-edge technology is set to enhance PPB’s quality control systems by detecting substandard and falsified medical products.”
The board revealed that the rollout began on Monday, December 9, at its headquarters and involved retraining personnel to operate the new system.
“The implementation includes personnel retraining conducted by software engineers from Global Health Labs USA, equipment qualification, and the development of a comprehensive library to support the technology’s operations,” PPB explained.
This development follows a recent directive from the board to quarantine a cancer drug, Flurasted 500 (5-Fluorouracil) Injection, Batch No. HHP24017. The action was taken after complaints were received regarding the appearance of the product’s contents.
PPB directed pharmaceutical outlets and health facilities to quarantine the affected batch to ensure patient safety and maintain public confidence in medical products.
This initiative is a step forward in strengthening Kenya’s health sector by ensuring only quality-assured medicines are available to the public.